Long-term safety and efficacy of ELZONRIS, the first and only targeted FDA-approved treatment for BPDCN, were evaluated in the long-term analysis, which included all patients from the initial analysis and those enrolled in the continued access cohort.1
Long-term efficacy of ELZONRIS in treatment-naive patients with BPDCN1,2
of patients (37/65) achieved CR/CRc*
- Primary endpoint
of patients who achieved CR/CRc (19/37)
bridged to SCT
- Secondary endpoint
>2 years
Median duration of CR/CRc
(95% CI, 3.8 to NR)
- Secondary endpoint
38.4 months
Median OS in patients who achieved CR/CRc and bridged to SCT (range, 3.4-58.1 months)
- Secondary endpoint
<40 days
Median time to CR/CRc
(range, 14-131 days)
- Secondary endpoint
Patients bridged to SCT a median of ~16 weeks after their first dose of ELZONRIS (range, 72-203 days)2
Overall survival for 1L patients by those bridged to SCT and response status3
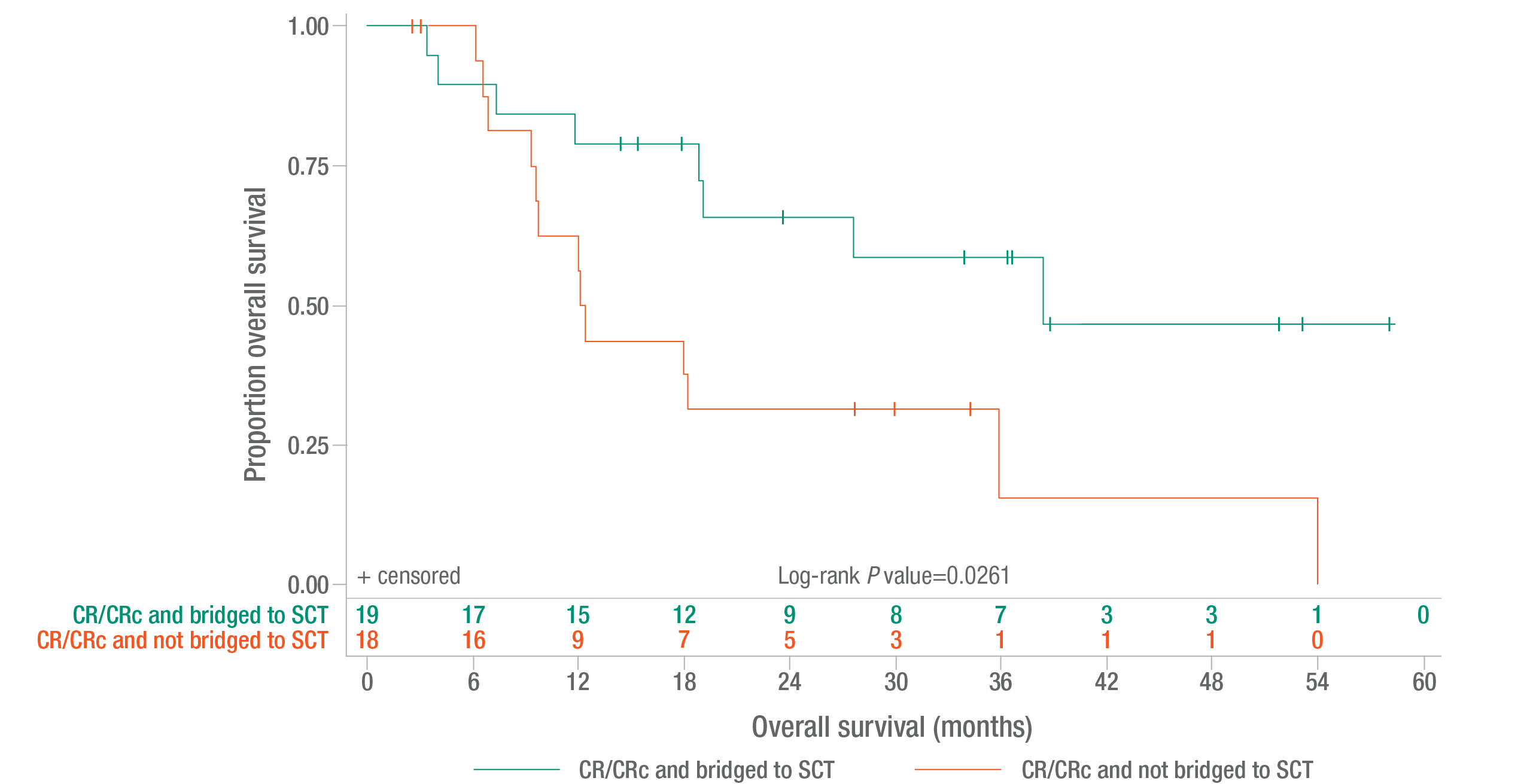

Overall survival was a secondary endpoint.2
Reprinted with permission from Pemmaraju et al. J Clin Oncol 2022 supplement.
Long-term efficacy of ELZONRIS in previously treated patients with BPDCN1,2
CR/CRc rate*
- Median duration of CR/CRc: 3.6 months (range, 3.0 to 13.9+ months)
- Primary endpoint
ORR
- Median time to reponse: 29 days
- Median duration of response: 2.9 months (range, 0.7 to 13.9+ months)
- Secondary endpoint
*CRc defined as complete response with residual skin abnormality not indicative of active disease.4
1L, first line; BPDCN, blastic plasmacytoid dendritic cell neoplasm; CI, confidence interval; CR, complete response; CRc, clinical complete response; NR, not reached; ORR, overall response rate; SCT, stem cell transplant.
- References:
- Pemmaraju N, et al. Long-term benefits of tagraxofusp for patients with blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2022;40(26):3032-3036.
- Data on file. Stemline Therapeutics, Inc.
- Pemmaraju N, et al. Long-term benefits of tagraxofusp for patients with blastic plasmacytoid dendritic cell neoplasm. J Clin Oncol. 2022;40(26):3032-3036 [supplementary appendix].
- ELZONRIS [prescribing information]. New York, NY: Stemline Therapeutics, Inc.; July 2023.




