Signs and symptoms of BPDCN
Signs and symptoms of BPDCN may be the same as many other illnesses, including other cancers. Common signs and symptoms of BPDCN may include swollen lymph nodes, stomach pain, and feeling tired. Eight to 9 out of 10 people with BPDCN may have skin lesions, a rash, or bruise-like marks on their skin.
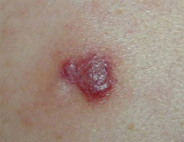
Nodular lesions
Nodular lesions, or an abnormal growth of tissue, can appear anywhere on the body. They are commonly found on the head, chest, back, arms, or legs.
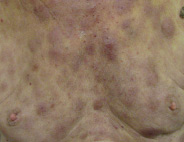
Bruise-like patches
One or more patches that look like bruises can appear anywhere on the body.
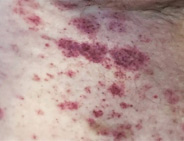
Red-brown patches
Discolored, usually flat, areas of skin that can appear anywhere on the body. They often don't feel itchy.
How BPDCN is diagnosed
THERE ARE DIFFERENT WAYS THAT HEALTHCARE PROVIDERS DIAGNOSE BPDCN.
They may biopsy skin, lymph nodes, or bone marrow. A biopsy means a healthcare provider will remove tissue from the skin, lymph nodes, or bone marrow, and look at it under a microscope.
One thing healthcare providers frequently look for in all of these tests is CD123, which is a type of protein found on BPDCN cells.
About ELZONRIS® (tagraxofusp-erzs)
ELZONRIS is a prescription medicine used to treat blastic plasmacytoid dendritic cell neoplasm (BPDCN) in adults and pediatric patients 2 years and older.
IMPORTANT SAFETY INFORMATION
ELZONRIS can cause serious side effects, including:
-
Capillary Leak Syndrome (CLS). ELZONRIS can cause fluid to leak from small blood vessels into your body's tissues. This is called "Capillary Leak Syndrome." CLS can quickly cause you to have symptoms that may become life-threatening or fatal (ie, lead to death). Get emergency medical help immediately if you develop any of the following symptoms:
- fast weight gain
- swelling of your face, arms, hands, legs, or feet
- shortness of breath or difficulty breathing
- low blood pressure (dizziness or lightheadedness, headache, feeling tired, or shortness of breath)
Weigh yourself daily. Your healthcare provider will also check your weight and test your blood before you receive each dose of ELZONRIS and as needed during treatment.
- Hypersensitivity reactions may occur with ELZONRIS. Symptoms may include rash, itching (pruritus), wheezing, or swelling in your face, including around your eyes and/or in and around your mouth
- Liver damage is usually detected through blood tests. Symptoms may include feeling tired (fatigue), loss of appetite, yellowing of your skin or the whites of your eyes (jaundice), or upper right abdominal pain or discomfort
Your healthcare provider will periodically test your blood while you are on ELZONRIS to check for liver damage.
Contact your healthcare provider immediately if you have any of these symptoms.
Getting medical treatment right away may help keep these problems from becoming more serious.
If you have any side effects during treatment with ELZONRIS, your healthcare provider may hold your treatment for a period of time or completely stop your treatment with ELZONRIS.
The most common side effects of ELZONRIS include CLS, nausea, feeling tired (fatigue), fever, swelling in your legs or feet, and weight gain.
These are not all of the possible side effects of ELZONRIS. If any new side effects start or an existing one gets worse, contact your healthcare provider immediately. For more information, talk to your treatment team.
Be sure to tell your treatment team about:
-
all of your medical conditions, including if you
-
are pregnant or plan to become pregnant. ELZONRIS may harm your unborn baby
- If you are a female who can become pregnant, you should use effective birth control during ELZONRIS treatment and for 1 week after the last dose
- Tell your healthcare provider right away if you become pregnant during treatment with ELZONRIS
- are breastfeeding or plan to breastfeed. It is not known if ELZONRIS passes into breast milk. You and your healthcare provider should decide if you will receive ELZONRIS or breastfeed. You should not do both
-
are pregnant or plan to become pregnant. ELZONRIS may harm your unborn baby
- all of the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements
You can report any side effects to Stemline Therapeutics, Inc. at 1-877-332-7961 or contact the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information, including Boxed WARNING, for ELZONRIS to learn more.
The risk information provided here is not comprehensive. To learn more, talk about ELZONRIS (tagraxofusp-erzs) with your healthcare provider or pharmacist. The FDA-approved product labeling can be found here.
